Research into development across the lifespan incorporates development from before birth to old age. The lifelong brain development community in Cambridge is made up of a diverse group of researchers working in a range of disciplines spanning the Departments of Biochemistry, Clinical Neurosciences, Chemistry, Chemical Engineering and Biotechnology, Genetics, Physiology, Development & Neuroscience, Paediatrics, Psychology, Psychiatry, Education and Zoology, as well as the Gurdon Institute of Developmental Biology, the MRC Cognition and Brain Sciences Unit, the Wellcome-MRC Cambridge Stem Cell Institute and the MRC Laboratory of Molecular Biology.
Teams of researchers working within and across departments and disciplines are investigating the development of the nervous system across the lifespan at a variety of levels and using a range of model systems, including embryos of different animal species (both vertebrate and invertebrate) and human cerebral organoids (‘mini -brains’). Questions currently being investigated range from how individual neurons form and arrange themselves into a nervous system, to how brain, behaviour and cognition develop across the lifespan, from prenatal through childhood and adolescence to old age. Research at Cambridge is also focused on brain mechanisms and cognitive development in developmental conditions such as autism and ADHD, in mental health problems such as depression, anxiety and psychosis, and in degenerative conditions such as dementia and Parkinson’s Disease.
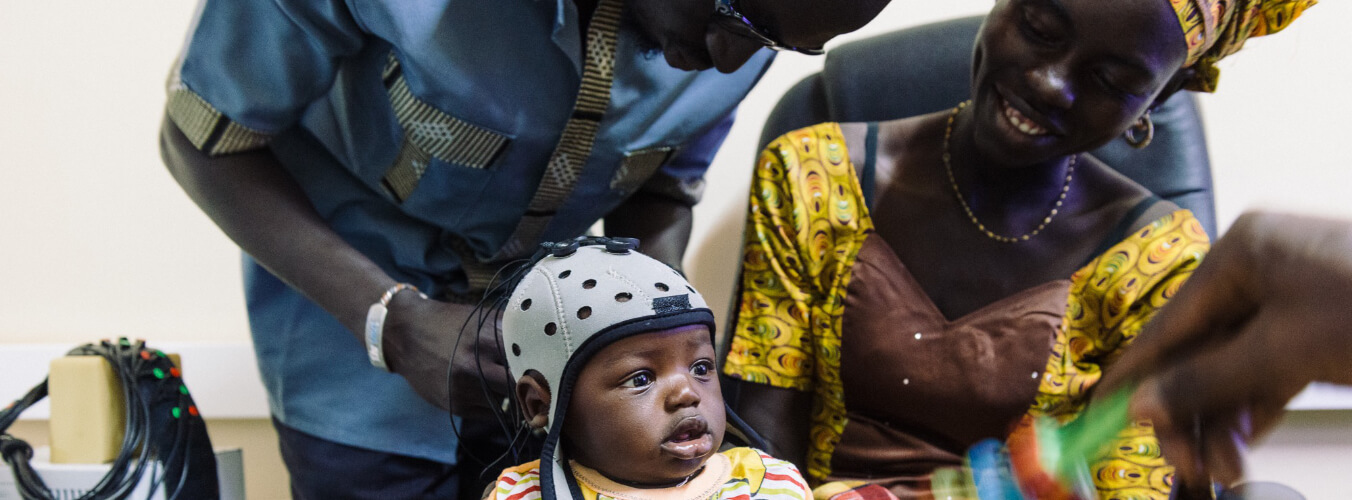
Research groups across Cambridge study brain and cognitive development in infancy, childhood and adolescence, using developmentally sensitive and appropriate tools and techniques – such as fNIRS, EEG and ultrasound to measure the infant brain, and MEG and MRI during childhood and adolescence. Carefully designed behavioural measurements allow research groups to study social cognition, attention, language, executive processes and memory, not only across the UK but in other countries including lower-middle-income countries (LMICs). Recently research has expanded to engage digital and online platforms. With the advent of the COVID-19 pandemic, a new focus has been on supporting families and understanding the impact of this pandemic, and related social and financial restrictions, on family life, health and infant, child and adolescent development.
Cross-disciplinary research at Cambridge focuses on the identification of the early changes and divergent trajectories that foreshadow learning difficulties and mental health disorders in young people, revealing opportunities for intervention and prevention. Neural markers of learning difficulties like dyslexia can be detected in infancy. Stress in development impacts the emergence, nature, and severity of mental illnesses, most of which first appear in adolescence. As with studies at other stages in the lifespan, datasets range from large population-based surveys and cohorts, to rare clinically identified groups, with many different methods applied to describe the phenotype of participants. For example, both the Centre for Attention, Learning and Memory (CALM) and the Centre for Neuroscience in Education (CNE) explore cognitive development over time in children with difficulties in learning, and with or without a formal diagnosis, in comparison to their peers. A hallmark of this science has been methodological innovations for understanding neural systems in childhood, how they are linked with developmental disorder, and how they respond to intervention.
Cambridge Children’s Hospital is a major new regional hospital and research institute with a core commitment to integrating physical and mental health that will open in 2025 on the Cambridge Biomedical Campus, Europe’s largest biomedical research cluster.
A wide range of interdisciplinary approaches are used to study the developing nervous system in a range of model organisms including fruit flies, frogs, zebrafish, chickens and mice, as well as human organoids. Questions currently being addressed include the cellular and molecular mechanisms underlying how cell polarity, cell movement and mechanics contribute to brain and spinal cord development, how stem cells self-renew or become specified as particular types of neurons or glia, how axons are guided to their targets and how neural circuits form and their properties are specified, including how synapses develop and are organised and how axons become myelinated. Some of these model systems are used to understand the biology of sensitive periods, which are stages of development characterised by increased vulnerability to stressors, which leave lasting impact on brain function.
Researchers are also studying the relationship between different kinds of mental stressors at different stages of postnatal brain development and the typical and atypical development of brains and behaviour from infancy, through adolescence to adulthood. By closely integrating studies in animals and humans important insights into the causes underlying the onset of mental health disorders can be revealed. This endeavour is facilitated by the newly built Translational Neuroimaging Laboratory (TNL) housing a 9.4T MRI and PET scanner in Cambridge, which is suitable for imaging the brains of rats, mice and a new world primate, the common marmoset. Already these studies are revealing points of overlap between the developing brain in humans and non-human animals. They are determining how changes in brain development relate to behavioural traits that are known risk factors for mental health disorders such as impulsivity, inflexibility and anxiety. They are also identifying how and when brain structures integrate across development to form the higher-order cognitive and emotional networks present in adults and the differential effects that stress at different stages of development may have on that integration.
Cambridge leads research on the cognitive neuroscience of health ageing. For example, the Cambridge Centre for Ageing and Neuroscience (Cam-CAN) brings together system neuroscientists and clinicians in the departments of Psychology, Psychiatry, Clinical Neurosciences, Engineering and Public Health. Based at the MRC Cognition and Brain Sciences Unit, Cam-CAN builds on a cohort of nearly 3000 adults, from 18-88 years of age, who have contributed demographic, lifestyle, cognitive, genetic and brain data (using MRI and MEG, N~700), resulting in important clues about how to maintain cognitive and brain health in old age. This complements long-standing work by Ageing Research Group of Cambridge Public Health, which has led population-based studies for decades, including the Cambridge City over 75 Cohort and the nationwide Cognitive Function and Ageing Studies. These have created multidisciplinary platforms for understanding fundamental aspects of brain ageing, including the neuropathologies of ‘common’ dementias and policy implications for public health.
The population cohorts complement our clinical studies, which focus on well characterised and deeply phenotyped (genetic, imaging, cognitive, biomarker) patient cohorts with and ‘at-risk of’ dementia and neurodegenerative disease. They aim to define the mechanisms of disease and develop novel imaging and biomarkers. These are translated into clinically relevant diagnostic markers and therapeutic approaches, which can then be widely applied. The studies are inherently collaborative with preclinical and clinical researchers from the Departments of Psychiatry, Clinical Neurosciences, Psychology, Mathematics, Chemistry, Engineering, MRC Cognition and Brain Sciences and others. We link closely to key partner NHS Trusts (Cambridge University Hospitals Trust and Cambridgeshire and Peterborough NHS Foundation Trust), the UK Dementia Research Institute, and Dementias Platform UK, and national NIHR infrastructure including the Clinical Research Network (CRN) and Join Dementia Research (JDR). Within the NIHR Cambridge Biomedical Research Centre our focus is on early stage translational studies, including novel repurposing studies and cell therapies.
The multi-site PREVENT dementia study of middle-aged people at risk of dementia is determining the role of genetic and lifestyle factors in progression of disease and defining imaging and other biomarkers at preclinical stages. As well as studying Alzheimer’s disease and vascular dementia, our work at the Cambridge Centre for Parkinson’s Plus, focusses on Progressive Supranuclear Palsy, Corticobasal degeneration, dementia with Lewy bodies, frontotemporal dementia and Parkinson’s disease. Researchers are investigating novel disease mechanisms, in particular the role of autophagy, protein synthesis and misfolding and neuroinflammation. The NIMROD study has played a key role in defining brain inflammation as a predictor of disease progression across several neurodegenerative diseases. Within the BRC and the renewed Dementias Platform these findings will be taken forward to determine the role of immune changes and synaptic function in early degenerative disease and to define and test new therapeutic approaches.
Recent advances in developmental neuroscience (e.g. imaging, connectomics) and computational science are increasingly generating large volumes of datasets in the context of population studies or brain and mental health disease-specific cohorts (e.g. dementia, mood-related disorders). Yet, processing and interpreting these large-scale data remains a key challenge for Translational Neuroscience. Artificial Intelligence approaches provide powerful and sensitive tools for synthesising and mining multimodal data across scales. Artificial neural networks inspired by brain function have surpassed major milestones in their effectiveness in complex tasks. These AI advances have the potential to advance our understanding of the interactive factors and early-life predictors of disease, revolutionise early and precise prediction, paving the way to personalised treatments. Intelligent systems based on machine learning approaches have the capacity to extract digital phenotypes (harvested from wearables and mobile devices) that in combination with clinical data (MRI scans, blood markers, genetics, medical records) can be used to improve diagnosis of brain and mental health disorders. In collaboration with the Alan Turing Institute, we are building AI-based solutions for precision brain and mental health with translational applications in neuroscience and clinical practice.
